
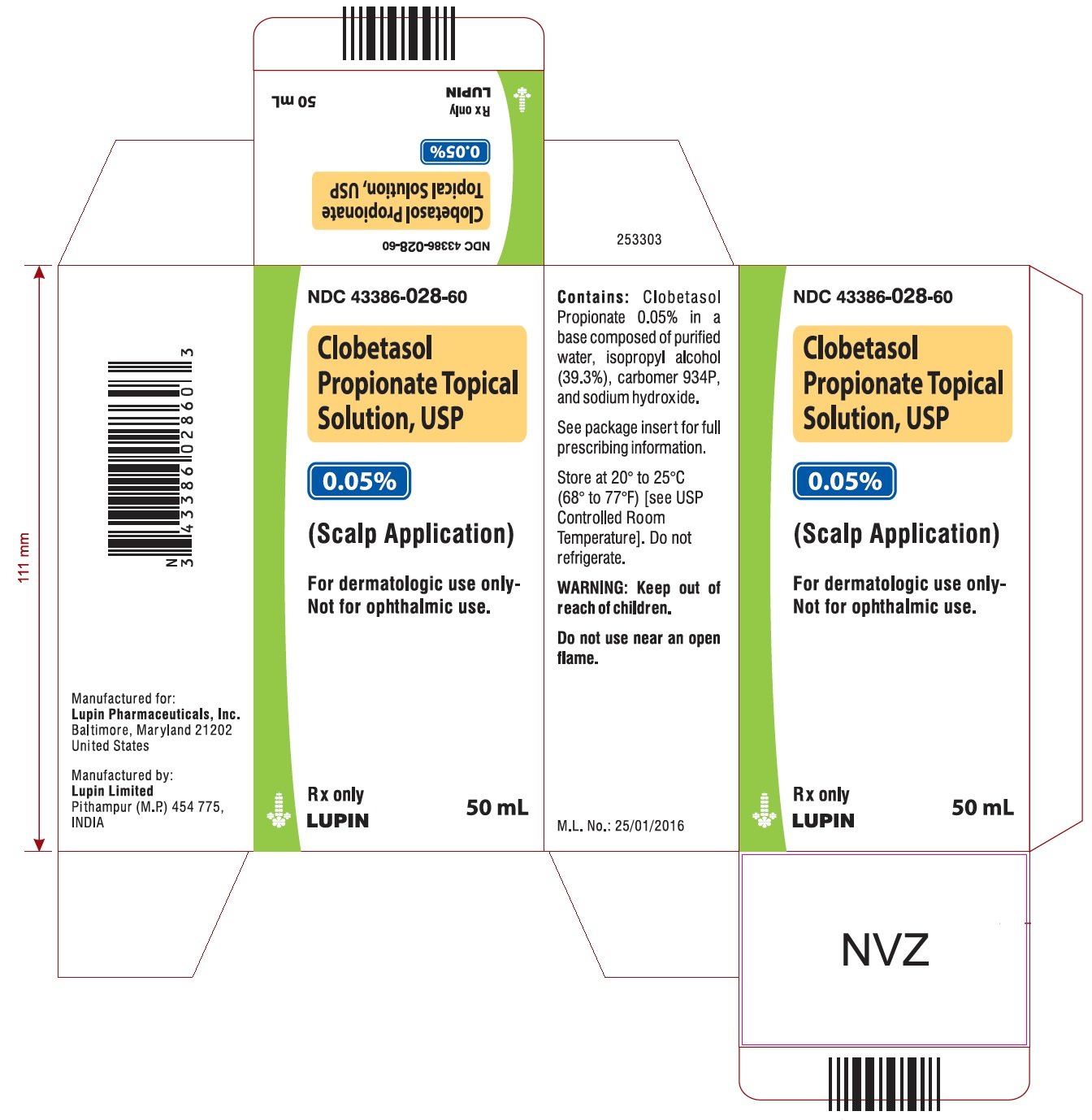
#CLOBETASOL TOPICAL SKIN#
Female subjects of childbearing potential must have a negative urine pregnancy test, must not be breastfeeding, and must agree to use an acceptable form of birth control for the duration of the study.Subjects with a normally functioning HPA axis, defined as a prestimulation serum cortisol level >5 µg/100 mL, and a response to cosyntropin stimulation to >18 µg/100 mL (after approximately 30 minutes) both blood draws for this test should be performed in the morning, if possible.Subjects with a clinical diagnosis of AD (according to the criteria of Hanifin and Rajka) of moderate to severe intensity (ISGA score of 3 or 4) involving ≥25% to ≤50% of total BSA located within treatable areas (Cohort 1), or ≥35% to ≤50% of total BSA located within treatable areas (Cohorts 2 and 3), with treatable areas including all but the face, axillae, groin, and scalp.Male or female subjects in good general health confirmed by medical history.HPA axis suppression is defined as a cortisol concentration ≤18 µg/100 mL at approximately 30 minutes after stimulation with cosyntropin. Enrollment into Cohorts 2 and 3 will proceed only if the percentage of subjects with HPA axis suppression in Cohorts 1 and 2, respectively, is ≤40%.

Enrollment into each successively younger pediatric cohort will proceed only after the preceding cohort has been completed and safety and exploratory data (including adverse events, tolerability assessments, clinical laboratory results, and the percentage of subjects with HPA axis suppression) have been reviewed and agreed to be acceptable for progression to the next cohort.
The study will consist of three successively younger pediatric cohorts, as safety data allow: This study is a multicenter, open-label study designed to evaluate the HPA axis suppression potential and systemic exposure to clobetasol, when administered as Clobetasol Topical Oil in pediatric subjects, under conditions consistent with anticipated clinical use and under conditions designed to maximize the potential for drug absorption in subjects with moderate to severe AD. Why Should I Register and Submit Results?.


 0 kommentar(er)
0 kommentar(er)
